Peking University, Feb. 28, 2011: A research group led by Professor Luo Jincai of PKU Institute of Molecular Medicine (IMM) recently discovered a critical role of “Gab1-PKA-eNOS” in the postnatal ischemic and VEGF-induced angiogenesis. The achievement was published in the January 31 issue of the Proceedings of the National Academy of Sciences (PNAS) titled “Grb-2-associated binder 1 (Gab1) regulates postnatal ischemic and VEGF-induced angiogenesis through the protein kinase A-endothelial NOS pathway."
Angiogenesis is a key step in the self-recovery of heart, brain, muscle, and other histoorgans after their hypoxia-ischemia. It plays an important role in facilitating blood circulation, preventing tissue injury and improving organ functions and so on. The intracellular signaling mechanisms underlying postnatal angiogenesis are incompletely understood. Endothelium-specific Gab1 KO (EGKO) mice displayed impaired angiogenesis in the ischemic hindlimb despite normal induction of VEGF expression. Matrigel plugs with VEGF implanted in EGKO mice induced fewer capillaries than those in control mice. The vessels and endothelial cells (ECs) derived from EGKO mice were defective in vascular sprouting and tube formation induced by VEGF. Biochemical analyses revealed a substantial reduction of endothelial NOS (eNOS) activation in Gab1-deficient vessels and ECs following VEGF stimulation. Interestingly, the phosphorylation of Akt, an enzyme known to promote VEGF-induced eNOS activation, was increased in Gab1-deficient vessels and ECs whereas protein kinase A (PKA) activity was significantly decreased. Introduction of an active form of PKA rescued VEGF-induced eNOS activation and tube formation in EGKO ECs. Reexpression of WT or mutant Gab1 molecules in EGKO ECs revealed requirement of Gab1/Shp2 association for the activation of PKA and eNOS. Taken together, these results identify Gab1 as a critical upstream signaling component in VEGF-induced eNOS activation and tube formation, which is dependent on PKA. Of note, this pathway is conserved in primary human ECs for VEGF-induced eNOS activation and tube formation, suggesting considerable potential in treatment of human ischemic diseases.
The paper was co-published by PhD candidates Lu Yao and Xiong Yan. The research was subsidized by Peking University's "985 Project” fund, National Natural Science Foundation, Department Science and Technology "973 Program," and the China National Key Laboratory's Independent Research Project Fund.
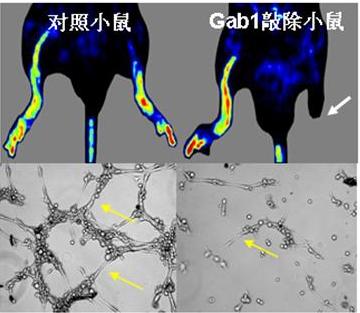
Endothelium-specific Gab1 KO mice displayed impaired angiogenesis in the ischemic hindlimb. The vessels and endothelial cells (ECs) derived from Endothelium-specific Gab1 KO were defective in vascular sprouting and tube formation induced by VEGF.
Translated by: Chen Wei
Edited by: Jacques
Source: PKU News (Chinese)