Peking University, May 15, 2011: An unusually unsymmetrical organometallic complex made up of an erbium atom sandwiched between two different aromatic hydrocarbon rings exhibits unique magnetic behavior, a new study shows. This complex could become a prototype for further development of single-molecule magnets (SMMs), which are being sought for applications such as high-density information storage and quantum computing.
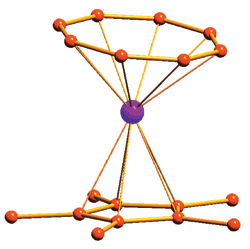
This erbium complex (hydrogens not shown) displays novel magnetic behavior for a single-metal-ion magnet.
Conventional magnets rely on the collective behavior of the unpaired electron spins of millions of individual metal atoms in a bulk material. SMMs, on the other hand, individually exhibit magnetlike behavior. A magnetic device made with these complexes, each storing a bit of data, could hold thousands of times more information than current storage devices.
Most SMMs are based on cluster compounds with multiple metal-ion cores such as Mn12O12, but only about 10 examples of single-metal-ion SMMs are known. A research team led by Gao Song and Wang Bingwu of Peking University has synthesized and studied the magnetic properties of a new type: an erbium cyclooctatriene pentamethylcyclopentadiene complex (J. Am. Chem. Soc., DOI: 10.1021/ja200198v).
The erbium complex is notable for being the first example of an organometallic single-metal-ion magnet; previous versions contain oxygen- or nitrogen-based ligands. In the complex, the C8 ring is closer to the erbium atom than the C5 ring, and the rings are not perfectly parallel to each other as they are in most other sandwich compounds.
Professor Gao and coworkers find that this orientation enables erbium’s electron configuration to generate magnetic properties not achieved before in SMMs: It has two thermal magnetic relaxation processes instead of one, and the magnetism exists at a higher temperature range.
“The field of single-molecule magnetism has been firmly rooted in coordination chemistry with oxygen, nitrogen, and cyanide ligands being the major players,” observes Kim R. Dunbar, an SMM specialist at Texas A&M University. “The discovery of a bona fide organometallic SMM brings the topic of magnetic bistability to a whole new synthetic audience. The field is now absolutely wide open for new developments.”
Edited by: Jacques
Source: College of Chemistry and Molecular Engineering