Peking University, Sept. 26, 2019: Recently, the group of Professor Guo Shaojun in College of Engineering, Peking University developed a novel type of sub-nanometer, highly curved PdMo nanosheets – due to its structural analogy with graphene, it was denoted as ‘PdMo bimetallene’– which showed extraordinary electrocatalytic performance towards the oxygen reduction reaction (ORR) in alkaline environment. When used as the cathode electrocatalysts, the PdMo nanosheets enables much enhanced changing/discharging performance in Zn-air and Li-air batteries. This work was published in Nature magazine on September 26th, 2019.
Fossil fuels has caused severe challenges in energy shortage, environmental pollution and climate change, thus urgently calling for the development of renewable clean energy technologies that enable a sustainable energy system. The storage and subsequent usage of the renewable yet intermittent energy sources, e.g. solar, wind etc., however, requires an electrochemical device that enables the interconversion of electricity and chemicals in an efficient manner. Of key importance to the operational efficiency of the device lies on the electrode-electrolyte interface, in which the desired electrochemical reactions occur as driven by a suitable electrocatalyst. Currently, the lack of high-performing electrocatalyst bottlenecks the penetration of renewable energy.
One of the biggest challenges in this field is the unfavorable kinetics of the ORR, and platinum group metals (PGMs)-based electrocatalysts are often required to improve the activity and durability. In the past decade, the ORR activities in acidic environment on platinum-based catalysts have been drastically improved via the tuning of alloying, surface strain and optimized coordination environment. Nevertheless, improving the activity of this reaction in alkaline media remains challenging due to the difficulty in achieving optimized oxygen binding strength on PGMs in the presence of hydroxide. In this study, PdMo bimetallene has been demonstrated to be an efficient and stable electrocatalyst for the ORR and the OER in alkaline electrolytes, and promising cathodic electrodes in Zn–air and Li–air batteries. The ultrathin feature of PdMo bimetallene enables an impressive electrochemically active surface area (138.7 m2/gPd) and a mass activity towards the ORR of 16.37 A/mgPd at 0.9 volts versus RHE in alkaline electrolytes. This mass activity is 78 times and 327 times higher than that of commercial Pt/C and Pd/C catalysts, respectively, along with negligible decay after 30,000 accelerated cycling. Density functional theory calculations show that an optimized oxygen binding energy was achieved on PdMo bimetallene due to a combination of alloying effect, strain effect and the quantum size effect. It is envisioned that the ‘metallene’ materials will show great promise in energy electrocatalysis.
Professor Guo Shaojun is the corresponding author of this paper. Collaborators include Professor Lu Gang from California State University and Dr. Su Dong from Brookhaven National Laboratory. This work was financially supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Beijing Natural Science Foundation, the BIC-ESAT project, the China Postdoctoral Science Foundation and others. The work at California State University Northridge was supported by the National Science Foundation PREM. The electron microscopy work used resources of the Center for Functional Nanomaterials, which is a US Department of Energy Office of Science Facility, at Brookhaven National Laboratory.
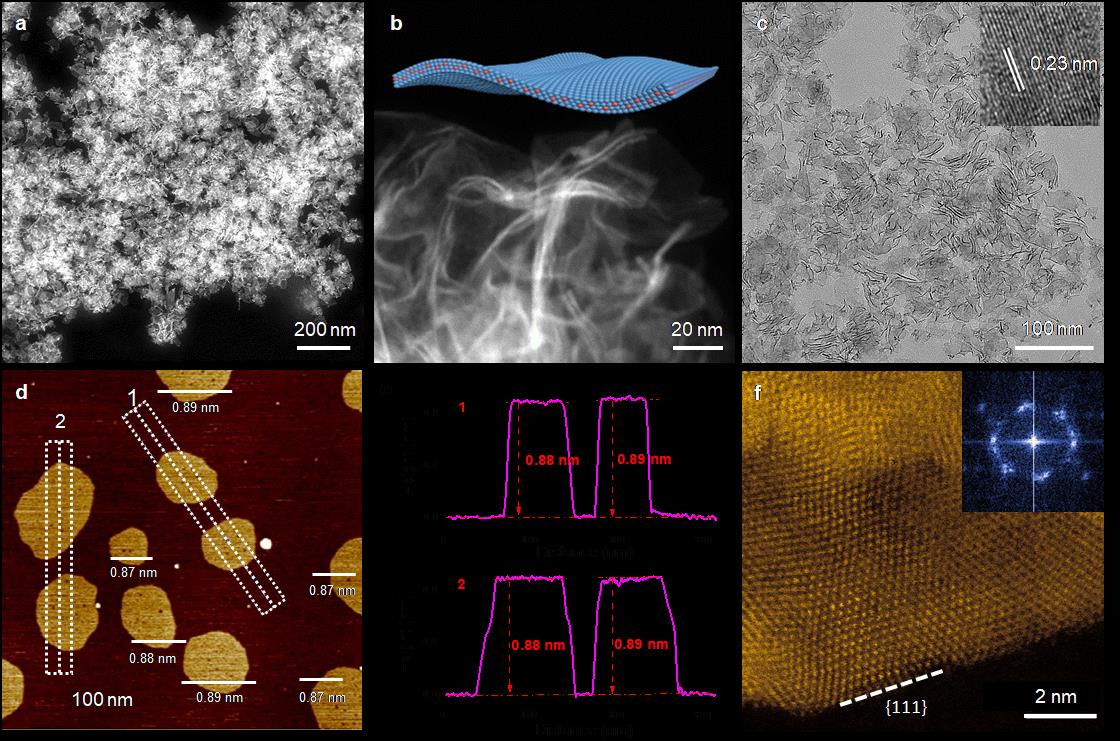
Fig. 1. Structural characterizations of PdMo bimetallene. a–c, Low-magnification HAADF-STEM (a), high-magnification HAADF-STEM (b) and TEM (c) images of PdMo bimetallene. The inset of c shows an HRTEM image of PdMo bimetallene. d, e, AFM image (d) and corresponding height profiles (e) of PdMo bimetallene. f, High-resolution HAADF-STEM image taken from a single bimetallene nanosheet. Inset, the corresponding fast Fourier transform patterns.
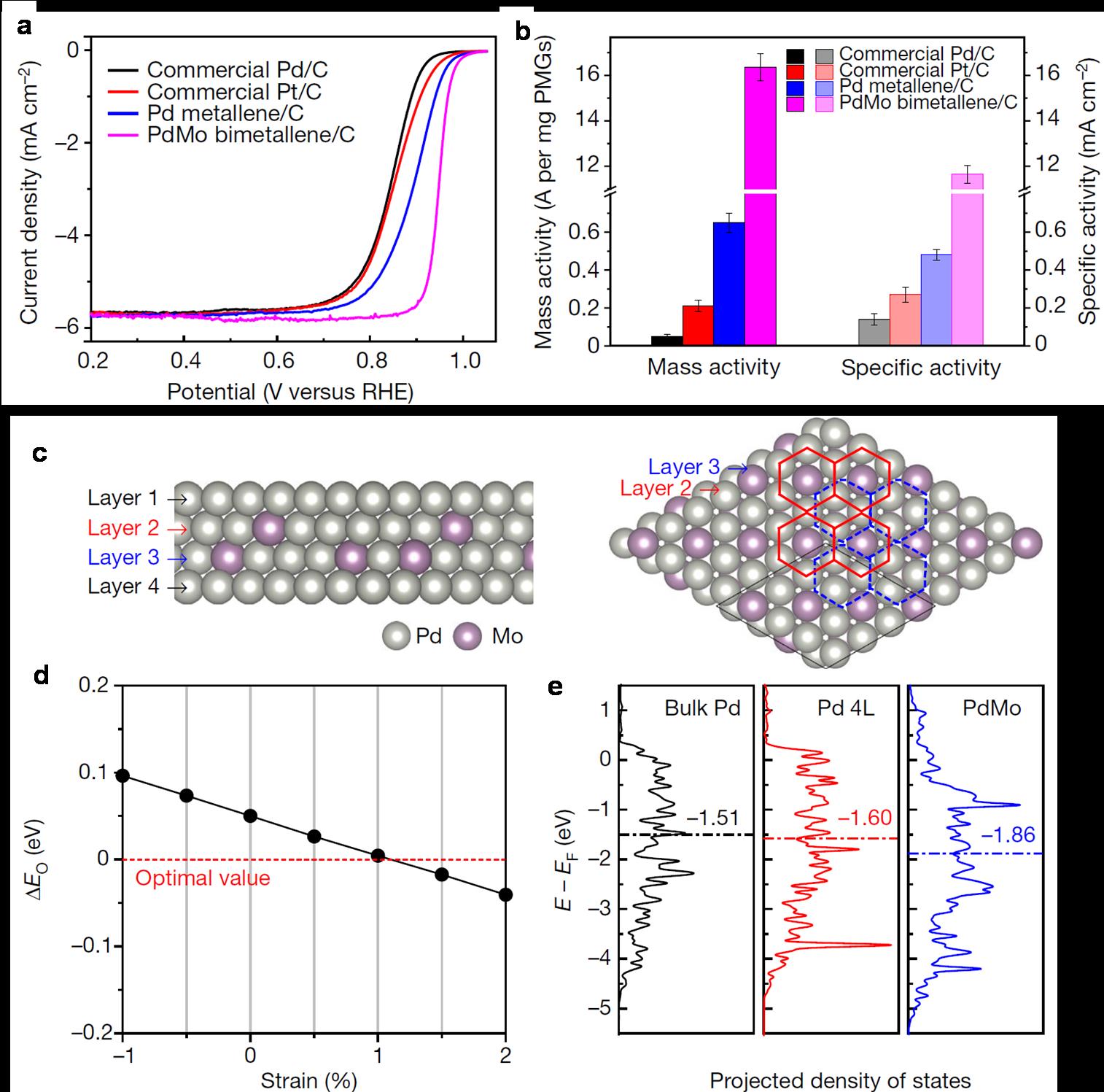
Fig. 2. Electrocatalytic performance and mechanism study. a, b, ORR polarization curves (a) and a comparison of the mass- and specific activities (b) of the stated catalysts in 0.1 M KOH at 0.9 V versus RHE. c, Left, side view of the atomic model of the four-layer PdMo bimetallene. Right, top view of the atomic model showing layers 2 and 3. In layers 2 and 3, each molybdenum atom is surrounded by six palladium atoms, indicated by the red (layer 2) and blue (layer 3) hexagons. d, Oxygen binding energy (ΔEO) of PdMo bimetallene as a function of compressive (negative) and tensile (positive) strains. The horizontal red line indicates the optimal ΔEO value. e, The projected electronic density of states of the d-band for the surface palladium atoms in bulk Pd, a four-layer Pd sheet (Pd 4L) and PdMo. The horizontal dashed lines indicate the calculated d-band centre.
Edited by: Huang Weijian
Source: College of Engineering

